Türk bilim insanları Okay Saydam ve Nurten Saydam kanser hücrelerinin, DNA onarımında görevli ve hücrenin hayatta kalması için kritik olan Ku protein kompleksinin kaybına nasıl uyum sağladığını öğrenmeye çalıştılar. Bilim insanları yeni çalışmaları ile kanser araştırmalarının yönünü değiştirmeyi hedefliyor.
ilk defa, kanser hücrelerinin nasıl parazit gibi diğer hücrelere grip yaşamaya çalıştığı gösterildi. Cumhuriyet.com.tr‘ye konuşan bilim insanları bu çalışmanın kanser araştırmalarının yönünü değiştireceğini söyledi.
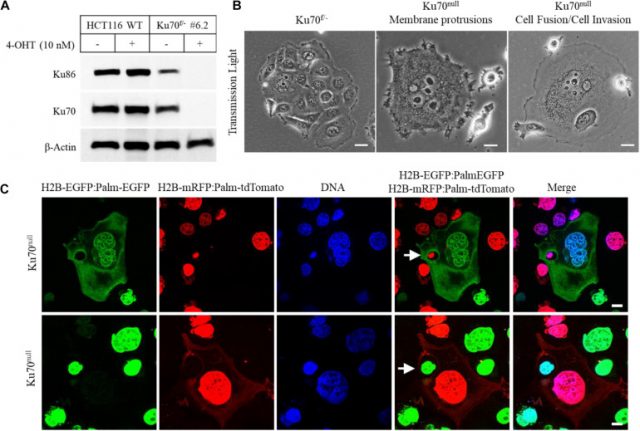
Araştırmacılar, kanser hücrelerinin, DNA onarımında görevli ve hücrenin hayatta kalması için kritik olan Ku protein kompleksinin kaybına nasıl uyum sağladığını öğrenmeye çalıştılar. Bu eksiklikle tasarlanmış bir kolorektal kanser hücre hattı kullanarak, Ku70 proteinini kademeli olarak devre dışı bırakarak Ku kompleksinin her iki bölümü olan Ku70 ve Ku86’nın tükenmesini sağladılar. Daha sonra, Ku’dan yoksun bu kanser hücrelerinin hareketini mikroskop altında incelediler ve aktivitelerini, Ku proteini içeren ana kanser hücreleriyle karşılaştırdılar.
Deficiency of Ku Induces Host Cell Exploitation in Human Cancer Cells
- 1Department of Pediatrics, University of Minnesota, Minneapolis, MN, United States
- 2Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota, Minneapolis, MN, United States
Cancer metastasis is the major cause of death from cancer (Massague and Obenauf, 2016; Steeg, 2016). The extensive genetic heterogeneity and cellular plasticity of metastatic tumors set a prime barrier for the current cancer treatment protocols (Boumahdi and de Sauvage, 2020). In addition, acquired therapy resistance has become an insurmountable obstacle that abolishes the beneficial effects of numerous anti-cancer regimens (De Angelis et al., 2019; Boumahdi and de Sauvage, 2020). Here we report that deficiency of Ku leads to the exploitation of host cells in human cancer cell line models. We found that, upon conditional deletion of XRCC6 that codes for Ku70, HCT116 human colorectal cancer cells gain a parasitic lifestyle that is characterized by the continuous cycle of host cell exploitation. We also found that DAOY cells, a human medulloblastoma cell line, innately lack nuclear Ku70/Ku86 proteins and utilize the host-cell invasion/exit mechanism for maintenance of their survival, similarly to the Ku70 conditionally-null HCT116 cells. Our study demonstrates that a functional loss of Ku protein promotes an adaptive, opportunistic switch to a parasitic lifestyle in human cancer cells, providing evidence for a previously unknown mechanism of cell survival in response to severe genomic stress. We anticipate that our study will bring a new perspective for understanding the mechanisms of cancer cell evolution, leading to a shift in the current concepts of cancer therapy protocols directed to the prevention of cancer metastasis and therapy resistance.
Deficiency of Ku Induces Host Cell Exploitation in Human Cancer Cells
- PMID: 33855027
- PMCID: PMC8040742
- DOI: 10.3389/fcell.2021.651818
Free PMC article
Abstract
Cancer metastasis is the major cause of death from cancer (Massague and Obenauf, 2016; Steeg, 2016). The extensive genetic heterogeneity and cellular plasticity of metastatic tumors set a prime barrier for the current cancer treatment protocols (Boumahdi and de Sauvage, 2020). In addition, acquired therapy resistance has become an insurmountable obstacle that abolishes the beneficial effects of numerous anti-cancer regimens (De Angelis et al., 2019; Boumahdi and de Sauvage, 2020). Here we report that deficiency of Ku leads to the exploitation of host cells in human cancer cell line models. We found that, upon conditional deletion of XRCC6 that codes for Ku70, HCT116 human colorectal cancer cells gain a parasitic lifestyle that is characterized by the continuous cycle of host cell exploitation. We also found that DAOY cells, a human medulloblastoma cell line, innately lack nuclear Ku70/Ku86 proteins and utilize the host-cell invasion/exit mechanism for maintenance of their survival, similarly to the Ku70 conditionally-null HCT116 cells. Our study demonstrates that a functional loss of Ku protein promotes an adaptive, opportunistic switch to a parasitic lifestyle in human cancer cells, providing evidence for a previously unknown mechanism of cell survival in response to severe genomic stress. We anticipate that our study will bring a new perspective for understanding the mechanisms of cancer cell evolution, leading to a shift in the current concepts of cancer therapy protocols directed to the prevention of cancer metastasis and therapy resistance.




 Okay Saydam
Okay Saydam Nurten Saydam
Nurten Saydam











