ARKA PLAN: Antikanser ilaçları için küresel ilaç satışları 74.4 $ (2014),
terapötik sınıfa göre tutar bazlı ilk sıradaki ilaç grubudur (ülkeye göre farklılık olabilir)
AMAÇ: Yeni antikanser ilaçları için onay ve kapsama kararlarını karşılaştırmak;
Amerika Birleşik Devletleri ve AB, İngiltere, Avustralya ve Kanada.
YÖNTEMLER: Onaylanan tüm yeni antikanser ilaç endikasyonlarını belirledik
(FDA tarafından 1 Ocak 2009 ve 31 Aralık 2013 arasında).
Her bir için ülke, organizasyonları, süreçleri, kriterleri ve özel değerlendirmeleri gözden geçirdik; ilaç için onay ve kapsama kararları vermek için kullanılan faktörler
göstergeler onaylandı.
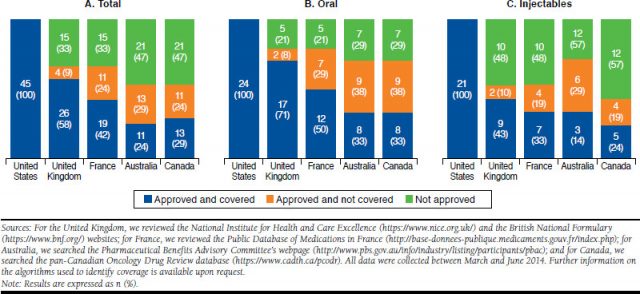
SONUÇLAR: Onay ve geri ödeme kararları önemli ölçüde ülkeye göre değişmektedir;
Amerika’nın en az erişim kısıtlaması vardı.
Avustralya, incelenen 5 ülkenin en kısıtlayıcı olanıydı.
TABLE 1 List of New Oncology Drugs Approved by the FDA, January 1, 2009-December 31, 2013
| Brand Name | Active Ingredient | Indication | Route of Administration | Approval Dates | Coverage | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FDA | EMA | Canada | Australia | United Kingdom | France | Canada | Australia | ||||
| Abraxane | Paclitaxel protein-bound particles | Non-small cell lung cancer | Injectable | October 2012 | Not Approved | Not Approved | Not Approved | NA | NA | NA | NA |
| Adcetris | Brentuximab vedotin | Hodgkin lymphoma and anaplastic large cell lymphoma | Injectable | August 2011 | October 2012 | February 2013 | December 2013 | Yes | Yes | Yes | No |
| Afinitor | Everolimus | Renal cell carcinoma | Oral | March 2009 | August 2009 | Not Approved | August 2009 | Yes | Yes | NA | Yes |
| Afinitor | Everolimus | Advanced pancreatic neuroendocrine tumors | Oral | May 2011 | September 2011 | August 2011 | July 2012 | Yes | Yes | Yes | Yes |
| Afinitor | Everolimus | Hormone receptor-positive, HER2-negative breast cancer | Oral | July 2012 | July 2012 | January 2013 | February 2013 | Yes | Yes | Yes | Yes |
| Arzerra | Ofatumumab | Chronic lymphocytic leukemia | Injectable | October 2009 | April 2010 | August 2012 | Not Approved | Yes | No | No | NA |
| Avastin | Bevacizumab | Renal cell carcinoma | Injectable | July 2009 | January 2008 | Not Approved | Not Approved | Yes | No | NA | NA |
| Bosulif | Bosutinib | Ph+ chronic myelogenous leukemia | Oral | September 2012 | March 2013 | Not Approved | Not Approved | Ye s | Ye s | NA | NA |
| Cometriq | Cabozantinib | Metastatic medullary thyroid cancer | Oral | November 2012 | Not Approved | Not Approved | Not Approved | NA | NA | NA | NA |
| Erivedge | Vismodegib | Basal cell carcinoma | Oral | January 2012 | July 2013 | August 2013 | May 2013 | Ye s | Ye s | Ye s | No |
| Erwinaze | Asparaginase Erwinia chrysanthemi | Acute lymphoblastic leukemia | Injectable | November 2011 | Not Approved | Not Approved | Not Approved | NA | NA | NA | NA |
| Folotyn | Pralatrexate | Peripheral T-cell lymphoma | Injectable | September 2009 | Not Approved | Not Approved | Not Approved | NA | NA | NA | NA |
| Gazyva | Obinutuzumab | Previously untreated chronic lymphocytic leukemia | Injectable | October 2013 | Not Approved | Not Approved | Not Approved | NA | NA | NA | NA |
| Gilotrif | Afatinib | Metastatic non-small cell lung cancer with EGFR mutations | Oral | July 2013 | September 2013 | Not Approved | November 2013 | No | Ye s | NA | No |
| Halaven | Eribulin mesylate | Metastatic breast cancer | Injectable | November 2010 | March 2011 | March 2012 | August 2012 | Ye s | Ye s | Ye s | No |
| He re eptin | Trastuzumab | Gastric cancer | Injectable | October 2010 | January 2010 | August 2010 | September 2010 | Yes | Yes | No | Yes |
| Iclusig | Ponatinib | Chronic myeloid leukemia and Philadelphia chromosome positive acute lymphoblastic leukemia | Oral | December 2012 | July 2013 | Not Approved | Not Approved | Yes | No | NA | NA |
| Imbruvica | Ibrutinib | Mantle cell lymphoma | Oral | November 2013 | Not Approved | Not Approved | Not Approved | NA | NA | NA | NA |
| Inlyta | Axitinib | Advanced renal cell carcinoma | Oral | January 2012 | September 2012 | August 2012 | July 2012 | Yes | Yes | Yes | No |
| Istodax | Romidepsin | Cutaneous T-cell lymphoma | Injectable | November 2009 | Not Approved | Not Approved | August 2013 | NA | NA | NA | No |
| Jevtana | Cabazitaxel | Prostate cancer | Injectable | June 2010 | March 2011 | August 2011 | December 2011 | Yes | Yes | No | Yes |
| Kadcyla | Ado-trastuzumab | HER2-positive metastatic breast cancer | Injectable | February 2013 | November 2013 | October 2013 | September 2013 | No | Yes | Yes | No |
| Kyprolis | Carfilzomib | Multiple myeloma | Injectable | July 2012 | Not Approved | Not Approved | Not Approved | NA | NA | NA | NA |
| Marchqibo | Vincristine | Ph-acute lymphoblastic leukemia | Injectable | August 2012 | Not Approved | Not Approved | Not Approved | NA | NA | NA | NA |
| Mekinist | Trametinib | Unresectable or metastatic melanoma with BRAF V600E or V600K mutations | Oral | May 2013 | Not Approved | August 2013 | Not Approved | NA | NA | No | NA |
| Perjeta | Pertuzumab | HER2+ metastatic breast cancer | Injectable | June 2012 | March 2013 | May 2013 | May 2013 | Ye s | Ye s | Ye s | No |
| Pomalyst | Pomalidomide | Relapsed and refractory multiple myeloma | Oral | February 2013 | August 2013 | Not Approved | Not Approved | Ye s | No | NA | NA |
| Provenge | Sipuleucel-T | Hormone refractory prostate cancer | Injectable | May 2010 | September 2013 | Not Approved | Not Approved | No | No | NA | NA |
| Revlimid | Lenalidomide | Mantle cell lymphoma | Oral | June 2013 | Not Approved | Not Approved | Not Approved | NA | NA | NA | NA |
| Stivarga | Regorafenib | Metastatic colorectal cancer | Oral | September 2012 | September 2013 | April 2013 | November 2013 | Ye s | No | No | No |
| Stivarga | Regorafenib | Gastrointestinal stromal tumor | Oral | February 2013 | Not Approved | April 2013 | Not Approved | NA | NA | No | NA |
| Sutent | Sunitinib | Pancreatic neuroendocrine tumors | Oral | May 2011 | December 2010 | Not Approved | March 2011 | Ye s | Ye s | NA | Yes |
| Sylatron | Peginterferon alfa-2b | Melanoma | Injectable | April 2011 | Not Approved | Not Approved | Not Approved | NA | NA | NA | NA |
| Synribo | Omacetaxine | Chronic or accelerated phase chronic myeloid leukemia | Injectable | October 2012 | Not Approved | Not Approved | Not Approved | NA | NA | NA | NA |
| Tafinlar | Dabrafenib | Unresectable or metastatic melanoma with BRAF V600E mutation | Oral | May 2013 | September 2013 | August 2013 | August 2013 | No | No | No | Yes |
| Vandetanib | Vandetanib | Thyroid cancer | Oral | April 2011 | February 2012 | February 2012 | January 2013 | Ye s | Ye s | No | No |
| Votrient | Pazopanib | Renal cell carcinoma | Oral | October 2009 | June 2010 | August 2010 | June 2010 | Yes | No | Yes | Yes |
| Votrient | Pazopanib | Soft tissue sarcoma | Oral | April 2012 | August 2012 | August 2010 | May 2011 | Ye s | No | No | Yes |
| Xalkori | Crizotinib | ALK+ non-small cell lung cancer | Oral | August 2011 | October 2012 | May 2012 | September 2013 | Ye s | Ye s | No | No |
| Xgeva | Denosumab | Giant cell tumor of bone | Injectable | June 2013 | Not Approved | June 2011 | Not Approved | NA | NA | No | NA |
| Xtandi | Enzalutamide | Metastatic castration-resistant prostate cancer | Oral | August 2012 | June 2013 | June 2013 | Not Approved | Yes | No | Yes | NA |
| Yervoy | Ipilimumab | Metastatic melanoma | Injectable | March 2011 | July 2011 | March 2012 | June 2011 | Yes | No | Yes | Yes |
| Zaltrap | Ziv-aflibercept | Metastatic colorectal cancer | Injectable | August 2012 | February 2013 | Not Approved | April 2013 | Yes | Yes | NA | No |
| Zelboraf | Vemurafenib | BRAFm+ melanoma | Oral | August 2011 | February 2012 | March 2012 | May 2012 | Yes | Yes | Yes | No |
| Zytiga | Abiraterone | Prostate cancer | Oral | May 2011 | September 2011 | July 2011 | March 2012 | Yes | Yes | Yes | Yes |
Sources: The FDA and CenterWatch websites were used to identify drugs approved by the FDA for the treatment of any cancer between the dates shown above (http://www.fda.gov/; http://www.centerwatch.com/).
Notes: The date December 31, 2013, was used as the end point for approval decisions, and June 30, 2014, was used as the end point for coverage decisions in non-U.S. countries. NA denotes not applicable because these drugs were not approved in other countries, so they were not covered.
EMA = European Medicines Agency; FDA =U.S. Food and Drug Administration.